On April 19, 2024, the Health Resources and Services Administration issued a final rule in the Federal Register titled “340B Drug Pricing Program; Administrative Dispute Resolution”.
Consistent with section 340B(d)(3) of the Public Health Service Act, the final rule establishes an Administrative Dispute Resolution (ADR) process to resolve:
- Claims by covered entities that they have been overcharged for covered outpatient drugs by manufacturers
- Claims by manufacturers, after the manufacturer has conducted an audit of a covered entity, that a covered entity has violated the prohibition on diversion or duplicate discounts
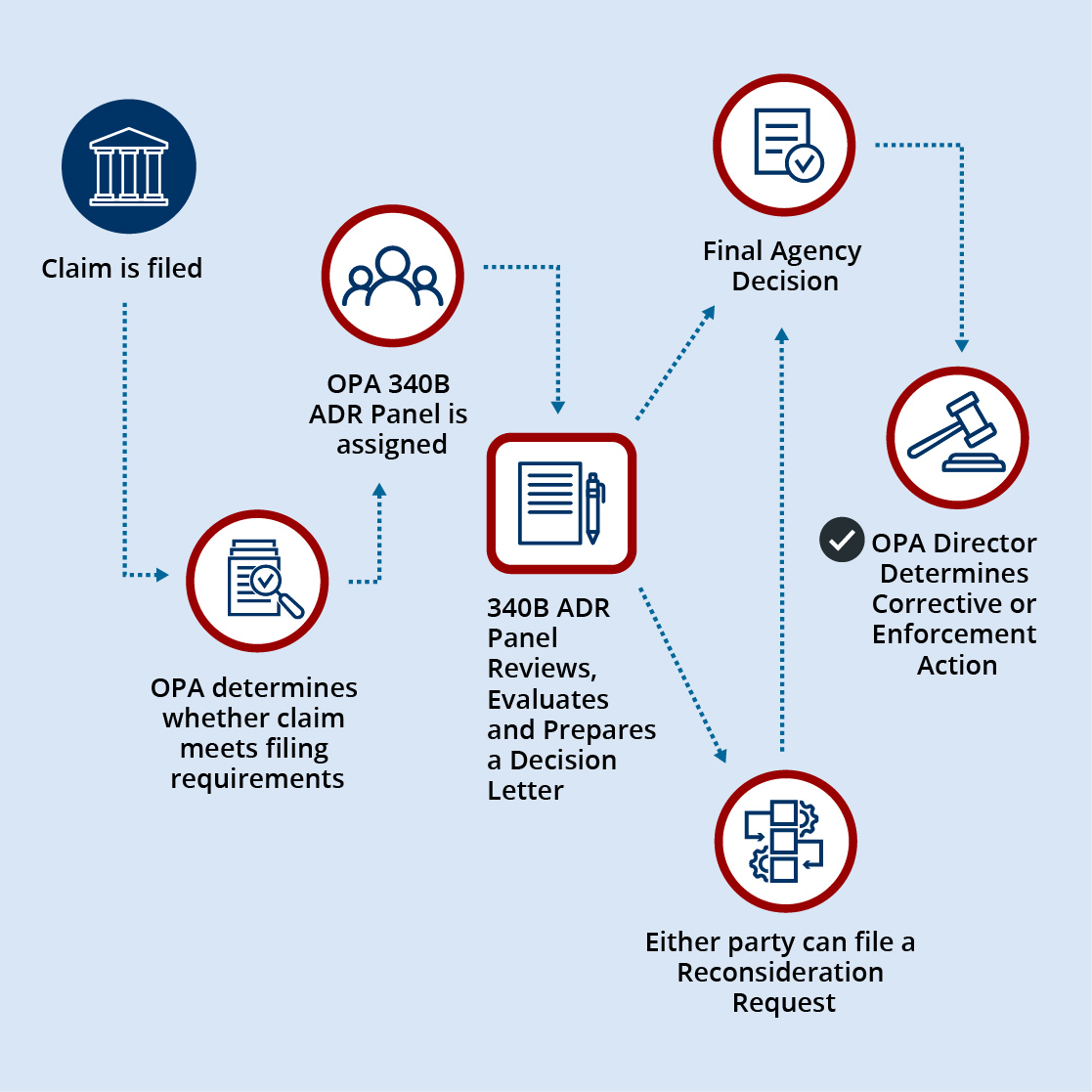
The 340B ADR final rule establishes a 340B ADR Roster consisting of members of HRSA’s Office of Pharmacy Affairs (OPA) staff who are appointed by the HHS Secretary. From the 340B ADR Roster, three members are selected to form a340B ADR Panel. Each 340B ADR Panel is selected and convened by the OPA Director. The 340B ADR Panel reviews properly submitted claims and issues final agency decisions. Parties to the claim will be offered an opportunity for reconsideration of the 340B ADR Panel’s decision.
The 340B ADR final rule requires documentation of good faith efforts to be submitted when filing a claim. 42 C.F.R. 10.21(b)(4). Covered entities and manufacturers should carefully evaluate whether the ADR process is appropriate given the investment of the time and resources required of the parties involved.
The 340B Office of Pharmacy Affairs Information System (340B OPAIS) will serve as the secure workspace for transferring and maintaining documents between parties. Specific steps about the ADR process are outlined in detail below. HRSA also encourages stakeholders to review the 340B ADR final rule for additional information regarding the submission process and timelines.
What are the steps in the ADR process?
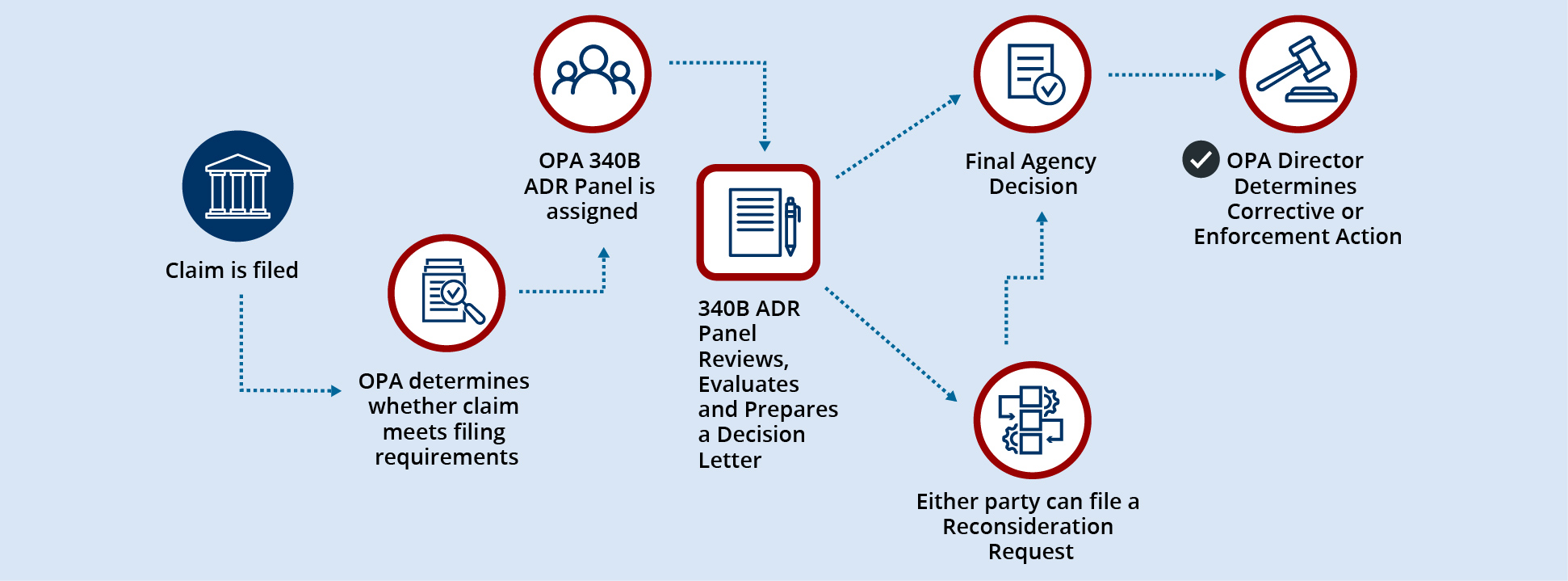
In general, the ADR process will involve the following steps:
- A petitioner emails HRSA at 340BADR@hrsa.gov with a request to file a claim through the ADR process. The email should include the petitioner’s 340B ID or Labeler Code, contact person’s name, email address, phone number, the opposing party’s 340B ID or labeler code, and a brief description of the reason the petition is being filed.
- The petitioner will receive an email from OPA with specific instructions on accessing the secure ADR workspace in 340B OPAIS, where the claim and supporting documents can be uploaded.
- Once the claim, including any supporting documentation, is submitted in 340B OPAIS, an OPA staff member will make a determination as to whether the requirements for filing a claim are met. The OPA staff conducting the initial review of a claim will not serve on the 340B ADR Panel reviewing that specific claim. OPA will notify the petitioner of whether the claim is deemed complete.
- If the claim is deemed complete, an OPA staff member will notify the opposing party of the claim’s status and provide instructions to a contact person of its choosing on how to access the 340B OPAIS ADR workspace and the deadline for responding to the claim (i.e., within 30 business days). If the opposing party fails to submit a response, the claim will proceed to the 340B ADR Panel for a decision based on the information available.
- At least three individuals will be selected from the 340B ADR Roster to form the 340B ADR Panel that will review the claim. Both parties will be notified of the selection of the 340B ADR Panel members, and that the claim has moved to the340B ADR Panel for review.
- The 340B ADR Panel will review the claim, the opposing party’s response (if any), and all supporting documentation or other information from the parties.
- Following its review, the 340B ADR Panel will issue a decision that will be sent to the parties and the OPA Director.
- Either party may request reconsideration within 30 business days of the date of the 340B ADR Panel’s decision. If reconsideration is requested, the 340B ADR Panel’s decision will be held in abeyance until the HRSA Administrator issues a reconsideration decision or declines to review the issue. A reconsideration decision will affirm or supersede the 340B ADR Panel decision.
- The OPA Director will determine any necessary corrective action or whether to take enforcement action, including referral to the HHS Office of Inspector General for its consideration of civil monetary penalties, as appropriate.
- Final agency decisions will be posted online in summary format within 120 calendar days of the issuance.
ADR process flow overview
Resources
340B Drug Pricing Program Administrative Dispute Resolution Final Rule Webinar (recorded on June 11, 2024)
FAQs
Following are some frequently asked questions (FAQs) related to 340B ADR process. If you have a question related to the 340B ADR process that is not covered by the information on this page or in the FAQs listed below, please submit your question to 340BADR@hrsa.gov.
When can parties begin submitting claims?
Stakeholders can begin submitting claims once the 340B Administrative Dispute Resolution (ADR) final rule (89 FR 28643, April 19, 2024) becomes effective on June 18, 2024. Detailed information on the submission process can be found on this webpage. Any additional questions can be sent to 340BADR@hrsa.gov.
Who will serve on the 340B ADR Panel?
The 340B ADR Panel will consist of individuals from a 340B ADR Roster comprised of OPA staff with subject matter expertise of the 340B Program. The OPA Director, or designee, will select at least three members for each 340B ADR Panel. Prior to appointment to the 340B ADR Roster, OPA staff will be screened for conflicts of interests by the OPA Director and government ethics officials. All individuals on the 340B Panel will also undergo additional screening conducted by HRSA prior to reviewing a specific claim.
How are the 340B ADR Panel members screened for conflicts of interest?
To be considered eligible for Secretarial appointment to the 340B ADR Roster, the OPA staff members must annually file and submit an Office of Government Ethics (OGE) Form 450 through the HHS Electronic Financial Disclosure System (EFDS). This form will also be certified by an Ethics Specialist in HRSA’s Office of Operations.
Prior to selection for an ADR Panel assignment, the OPA Director or designee, will evaluate if a prospective Panel member has any potential conflicts, including financial interest conflicts, current or former business relationships or other involvement with parties involved in the claim.
Additionally, the OPA Director or designee will perform screening to ensure that a 340B ADR Panel member was not directly involved in a decision concerning the specific issue of the ADR claim as it relates to the specific covered entity or manufacturer involved, including previous 340B ADR Panel decisions.
The Secretary may remove any individual from the 340B ADR Roster for any reason, including from any 340B ADR Panel to which the individual has already been assigned. To the extent that any significant conflict issue is raised after appointment, the OPA Director or the Secretary have the discretion to remove a 340B ADR Panel member.
What type of claims are to be submitted for review by the 340B ADR Panel?
In accordance with the 340B ADR final rule (89 FR 28643, April 19, 2024), claims may be submitted by:
- Covered entities that may have been overcharged for covered outpatient drugs purchased from manufacturers
- Manufacturers of 340B drugs, after the manufacturer has conducted an audit of the covered entity, when covered entities may have violated the prohibitions against duplicate discounts or diversion
In addition, the petition must be submitted within three years of the date of the alleged violation. Stakeholders must also submit documentation of any prior good faith efforts to resolve the dispute at issue.
What is the monetary threshold for filing an ADR claim?
There is no monetary threshold that must be satisfied for a claim to proceed to the 340B ADR Panel review. However, covered entities and manufacturers should carefully evaluate whether the ADR process is appropriate for minor or de minimis claims given the investment of time and resources required of the parties involved.
What type of information is needed from stakeholders prior to submitting a petition through the ADR process?
Covered entities and manufacturers must attempt to resolve issues in good faith prior to initiating a formal ADR process, which should be used as a last resort. Covered entities and manufacturers should carefully evaluate whether the ADR process is appropriate given the investment of the time and resources required of the parties involved.
When submitting a claim, stakeholders must include any documentation of prior good faith efforts, along with documentation including invoices, audit reports, policy statements, or correspondence, to substantiate the claim filed by the petitioner.
Stakeholders should email the following information to 340BADR@hrsa.gov to begin the 340B ADR process:
- Petitioner Organization Name, 340B ID or Labeler Code
- Petitioner Point of Contact Name, Email Address and Phone number
- The opposing party’s 340B ID or Labeler Code
- A brief (1-2 sentence) description of the reason the petition is being filed.
How long is the ADR process expected to take?
The 340B ADR Panel’s decision letter will be completed within one year of receiving a complete claim for review. Depending on the complexity of the issue, issuance of the ADR Panels decision letter may occur after this date.
How does the 340B ADR Panel formulate the final agency decision?
The 340B ADR Panel will review the claim, the opposing party’s response, and supporting documentation or other information from the parties. The 340B ADR Panel will follow the 340B statue, regulations, and all policies governing the 340B Program when reviewing and evaluating ADR claims. Following its review of all evidence, the 340B ADR Panel will issue a decision that will be sent to the parties and HRSA.
Within 30 business days of the 340B ADR Panel decision, manufacturers or covered entities may initiate a reconsideration request. If neither party submits a reconsideration request, then based on the final decision the OPA Director will determine any necessary corrective action or whether to take enforcement action, including referral to the HHS Office of Inspector General for its consideration of civil monetary penalties, as appropriate.
Can organizations or associations representing covered entities file an ADR action?
Yes. Covered entities must be members of the organization or association filing a claim on their behalf. All claims must allege violations by the same manufacturer and for the same drug(s). Organizations or associations must submit a joint claim attestation letter that they have confirmed that all the individual covered entities have agreed to be part of the ADR claim.
Can claims be combined for covered entities?
Joint claims are permitted for covered entities if certain criteria are met. Multiple covered entities, or their membership organizations or associations, can file together against a single manufacturer for the same drug(s) (a joint claim), but each must submit all documentation required to file a claim (e.g., invoice, 340B ceiling price, attempts to purchase at 340B). A joint claim attestation letter that confirms each covered entity has agreed to be part of the ADR claim must also be submitted.
Can claims be combined for manufacturers?
Multiple manufacturers can file together against a single covered entity (a consolidated claim) if each manufacturer could individually file a claim against the covered entity, consents to the consolidated claim, meets the requirements for filing a claim, and the 340B ADR Panel determines that such consolidation is appropriate and consistent with the goals of fairness and economy of resources.
Consolidated claims filed on behalf of manufacturers by associations or organizations representing their interests are not permitted.